RESEARCH
'Confinement-controlled Chemistry' is a common factor in outstanding fundamental scientific problems in fields such as energy technology, environmental studies, catalysis, and biomedicine. Confinement may also influence environmental issues, for example in ion selective membranes for desalination, morphologically controlled heterojunctions in photovoltaics, electrodes for battery materials in energy technology, or selective ion receptors in nuclear waste. In catalysis artificial enzymes or porous solids are used. In GRK 2376, state-of-the-art experiments and theoretical models are employed to explore how confinement to nanoscale dimensions alters the transition states and pathways of chemical reactions.

The collaborative research will determine how the nature of the confining environment, e.g. surface chemistries, surface roughness, or geometry (2D vs. 1D vs. 0D), can ultimately be used to control key features that guide important chemical transformations. For this, a range of new experimental and theoretical tools is developed and applied in order to address the fundamental problem of 'Confinement-controlled Chemistry': the complementary scientific approach will combine supra-molecular chemistry, fast laser spectroscopy, synchrotron X-ray spectroscopy, linear and nonlinear vibrational spectroscopies, single molecule imaging, and theoretical techniques in quantum chemistry, force field and ab initio molecular dynamics and free energy simulations.
The following research projects are conducted by current and former members of the Graduate School GRK 2376:
Principal Investigator Guido Clever
Selective Recognition and Reactivity in Nano-Confinements
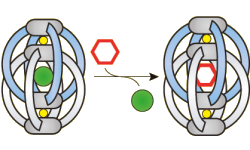
The aim of the project is to develop and refine tailor-made environments for selective guest recognition and chemical transformations within nano-sized, supramolecular self-assemblies. Following established assembly strategies, these nano-confinements are equipped with various functions in their "walls" such as non-covalent recognition sites, chiral elements and catalytic groups. A family of modular structures, featuring different sizes and functionalities, will form the basis to screen substrate uptake, conversion and product release. Optimization studies will focus on chemical stability, selectivity and reaction kinetics. Synthetic studies will be complemented by training in a wide range of analytical techniques such as UV/Vis-, fluorescence-, and NMR-spectroscopy, ion mobility mass spectrometry, isothermal titration calorimetry, cyclic voltammetry and X-ray crystallography.
PhD students: Alexandre Walther, Ertuğrul Yalçın
2nd supervisors: Axel Rosenhahn, Lars Schäfer
Confinement in Supramolecular Cages
The aim of the project is to determine the propensity for guest binding and ability to promote encapsulated chemical reactions of organic cages. Among the studied compounds are switchable Pd2L4 cage and Pd2L3 bowl systems or photoswitchable diarylethene Pd2L4 cages for which guest exchange dynamics are of special interest. A further goal is the exploration of neutral guest binding interpenetrated Pd4L8 cages for investigating light-initiated radical reactions. The synthetic methods are complemented by UV/Vis, fluorescence, and NMR spectroscopy, HR-ESI-MS, ion mobility mass spectrometry, isothermal titration calorimetry, cyclic voltammetry, X-ray crystallography, and DFT calculations.
PhD students: Kristina Ebbert, André Platzek, Elie Benchimol
2nd supervisors: Christian Merten, Karina Morgenstern
Principal Investigator Martina Havenith
Chemistry in Confinement Characterized by THz Spectroscopy
The aim of the project is to study reactions in nanoconfinement and nanoenclosures by THz spectroscopy, identifying the low frequency fingerprints of pressure-induced spectral changes of trapped molecules and the changes of Thz dynamics of confined water. Stability and reactions under confinement are studied as a function of pore size and/or layer distance (e.g. pressure-assisted dissociation of salts into their hydroxides and oxides) for materials like graphene, silica structures, or organic sub-nanopores as nanoreactors. The applied experimental techniques are THz-FTIR spectroscopy for pressure- and temperature-dependent studies, non-linear THz spectroscopy, and nano-electrospraying for salts solutions in confinement.
PhD students: Sarah Funke, Thorsten Ockelmann
2nd supervisors: Dominik Marx, Wolfram Sander
Characterization of the encapsulation process in nanocages by THz and dielectric spectroscopy
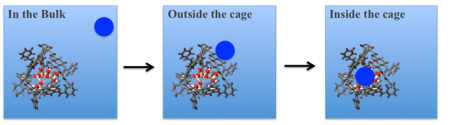
We want to investigate nanocages with internal cavities that serve as catalysts for host molecules. It turns out that the inner electric fields as well as the solvent phase facilitate the catalytic activity. The goal is now to shine light on the structure and dynamics of the solvent in a well-known supramolecular assembly at ambient conditions and quantify the thermodynamic properties, which are different to any known phase of water. Both the entropic unfavourable solvent configuration as well as the internal electric fields facilitate catalysis: Essentially, inside the supramolecular cage, are favouring the entrance of guest molecules. This process can be characterized by THz spectroscopy.
PhD students: Sanjana Nalige, Melinda Nolten
2nd supervisors: Wolfram Sander, Christian Merten
Principal Investigator Christian Merten
Optical Activity in 0D–confined Spaces
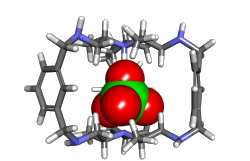
The aim of the project is to understand the mechanism of chiral induction from chiral organic cage molecules to encapsulated guests. We study this important first step towards reactions in molecular cages using model systems such as chiral cryptands. Within the project, the focus is on the synthesis of these model systems and their spectroscopic characterization. Besides the design of novel supramolecular chiral cages compounds, we utilize solution-phase vibrational circular dichroism to characterize the stereochemistry of the cages and the host-guest complexes.
PhD students: Frederike Beyer, Clemens Müller
2nd supervisors: Martina Havenith, Guido Clever
Optical Activity in 0D–confined Spaces
The aim of the project is to gain insight into the importance of the chemistry of the walls onto the induction of optical activity into the vibrational modes of small chiral guest molecules. This is investigated for organic cages composed of achiral side-arms and chiral head groups by solution-phase vibrational circular dichroism. This spectroscopic method is sensitive for chiral molecular structures and intermolecular interactions and is employed in combination with DFT-based spectra calculations and molecular dynamics simulations for the analysis of experimental data. Suitable organic model compounds are synthesized within the project.
PhD student: Kevin Scholten
2nd supervisor: Guido Clever
Can the Chiral Shape of a Confined Space Determine Stereoselectivity?
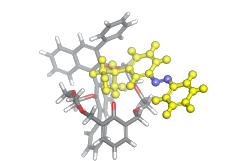
The aim of the project is to gain spectroscopic insights into conformational preferences of small guest molecules in macrocycles in order to elucidate how the chiral host molecules influence chiral reactions taking place in or at the guest molecule. Studies include chiral crown ethers and other macrocyclic compounds in solution and photochemical isomerization as model reactions. Vibrational circular dichroism experiments are performed in solution and are complemented by DFT-based spectra calculations and molecular dynamics simulations, as well as organic synthesis of the investigated model compounds.
PhD student: Luisa Weirich
2nd supervisor: Lars Schäfer
Principal Investigator Karina Morgenstern
Disentangling One- and Two-dimensional Confinement Effects from Wall Effects
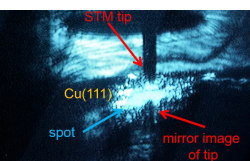
The aim of the project is to determine the effect of the dimensionality of confinement onto elementary surface reactions and disentangle geometrical confinement effects from changes due to electronic alterations of the molecules on a fundamental level. Laser-induced photochemical reactions are studied on patterned surfaces (NaBr, AAO and rare gas layers) or within organic cages adsorbed on metal surface. The molecules comprise carbenes, phosphines, and phthalocyanines. Several scanning tunneling microscope techniques are applied, e.g. at low temperature in ultrahigh vacuum or with meV resolution, complemented by in-situ molecule deposition and laser activation as well as Low-temperature infrared spectroscopy under UHV conditions with sub-meV resolution.
PhD students: Iheb Baklouti, Yong-He Pan
2nd supervisors: Wolfram Sander, Kristina Tschulik
Disentangling One- and Two-dimensional Confinement Effects from Wall Effects
The aim of the project is to determine the effect of the dimensionality of confinement onto elementary surface reactions and disentangle geometrical confinement effects from changes due to electronic alterations of the molecules on a fundamental level. Laser-induced photochemical reactions are studied on surfaces (ZnO and rare gas layers on a patterned surface) for azobenzene and carbene precursors with small inorganic molecules. Several scanning tunneling microscope techniques are applied, e.g. at low temperature in ultrahigh vacuum or with meV resolution, complemented by in-situ molecule deposition and laser activation as well as infrared spectroscopy under UHV conditions at 80 K with sub-meV resolution.
PhD students: Inga Langguth, Vladimir Lykov
2nd supervisors: Wolfram Sander, Dominik Marx
Principal Investigator Axel Rosenhahn
Ion Coordination, Nanoparticle precipitation, and Polymerization reactions in Reverse Micelles Studied by X-ray Analysis
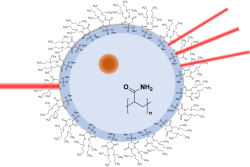
The effect of spatial confinement on nanoparticle precipitation and polymerization reactions will be investigated by spectroscopy and scattering techniques. The encapsulation of the aqueous phase within a reverse micelle provides a confined reaction volume with properties that are determined by their size and the used surfactant. Metal ion coordination, nanoparticle precipitation, and polymerization reactions inside the confined nanoreactors will be investigated by ATR-FTIR spectroscopy, dynamic light scattering, scanning electron microscopy, and UV-Vis spectroscopy. In addition, selected synchrotron-based X-ray analysis techniques including small angle X-ray scattering and X-ray spectroscopy will be applied.
PhD student: Samantha Muhring-Salamone
2nd supervisor: Kristina Tschulik
Ion Coordination and Precipitation Reactions in Reverse Micelles Studied by X-ray Analysis
The aim of the project is to determine the effect of spatial confinement on ion coordination in solution and its effect on nanoparticle precipitation reactions. The confinement is realized by reverse micelles with enclosed small ions. Metal ion coordination and nanoparticle precipitation induced by reducing agents and by electrochemical means are investigated by various spectroscopic methods. Among these are soft X-ray scattering (performed at storage ring beamlines) in a chamber with cryogenic samples environment and liquid jet technology, small angle X-ray scattering, and step-scan FTIR with stopped flow.
PhD students: Patricia Gnutt, Lisa Schardt
2nd supervisor: Kristina Tschulik
Principal Investigator Lars Schäfer
Towards QM-based machine learning potentials for supramolecular metallacages and enzymes
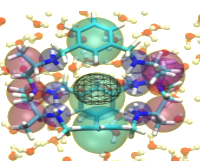
The use of machine learning for developing quantum mechanics-based potential energy functions that can describe chemical reactions and are at the same time computationally efficient is revolutionizing chemistry.
In this computational chemistry PhD project, efficient and accurate potentials will be developed and applied to study confinement effects in metal-containing systems, such as supramolecular metallacages and metalloenzymes. The project is carried out in collaboration with the group of Fernanda Duarte (Oxford University).
PhD students: Anna Selina Juber, Gers Tusha, Lucas Araujo de Lima Dias
2nd supervisors: Guido Clever, David Van Craen, Marialore Sulpizi
Principal Investigator Marialore Sulpizi
Understanding the role of micelle nanoconfinement on the electrochemical synthesis of metallic nanoparticles from multiscale molecular dynamics simulations
Synthesis of nanomaterials with desired shape and size is very important for their potential applications in catalysis, sensing, plasmonics. The reverse micellar method allows to control size and morphology through the micelle cavity diameter. In this method reverse micelles are formed by two non-miscible components and a surfactant which is characterized by amphiphilic properties. The electrochemical synthesis of metallic gold nanoparticles using micelle confinement has been experimentally achieved and is currently under investigation e.g. in sodium dodecyl sulphate in toluene and HAuCl4 solutions with and without HBF4. The confined nanoreactor in the micelle cavity provide a unique environment whose properties can be exploited to obtain control not only on morphology, but also in mass distribution to obtain alloy or core-shell nanoparticles. To advance the tailored synthesis a microscopic understanding of the confined nanoreactor environment is a key step. In this project we aim to use multiscale molecular dynamics simulations to address the following questions: (i) what is the micelle structure and different molecular species distribution? (ii) what is the role of the cations and anions in the solutions and how charges species can be used to tune shape and morphology? (iii) what happens when the micelle is adsorbed on the electrode during reduction?
PhD student: Qixuan Li
2nd supervisor: Kristina Tschulik
Impact of a liquid crystal columnar confinement of nucleotides on enhancing polymerization reactions
When exploring the origin of life, one main question remains open: how did we get from single nucleotides to long RNA and DNA chains which then led to more complex biological structures, following the RNA world hypothesis. More specifically, we are interested in how the nucleotides interactions are able to promote the synthesis of long polynucleotides.Experimental studies suggest that free nucleotides in water spontaneously organize into small molecular columnar phases, promoting the ligation of nucleic acid chains. To uncover the mechanisms behind this self-assembly, we use Molecular Dynamics simulations combined with Machine-Learning approaches.
Ab initio methods are too computationally expensive for the timescale of interest and for the complexity of the investigated systems. To overcome this limitation, we use Neural Network Potentials (NNPs) trained with DeepMD-kit and reinforced by metadynamics. After mastering the static and dynamical properties of a single Adenosine Monophosphate (AMP) in water, we are now investigating the stacking and pairing interactions between several AMPs by predicting the free energy landscape of this system as a function of the relevant degrees of freedom.
PhD student: Laurie Stevens
2nd supervisor: Axel Rosenhahn
Principal Investigator Kristina Tschulik
Altered Electrochemistry in 0D Confinement
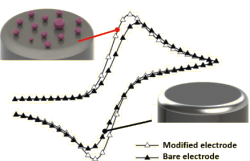
The aim of the project is to determine the effects of geometric confinement in 0D on charge transfer at the solid/liquid interface. Using single micelle electrochemistry and ensemble studies, the reactivity changes of solution phase redox couples in 0D confinement are explored, and the effect of 0D confinement on electrochemical reaction rates and mechanism of multi-electron transfer reactions is investigated. Applied experimental techniques comprise cyclic voltammetry and chronoamperometry at micelle modified electrodes, single entity electrochemistry, and surface and micelle characterization (AFM, dark field microscopy & hyperspectral imaging).
PhD student: Maximilian Jaugstetter, Kevin Wonner
2nd supervisor: Axel Rosenhahn
Altered Electrochemistry in 1D Confinement
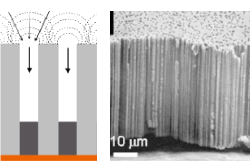
The aim of the project is to determine the effects of geometric confinement in one dimension on charge transfer at the solid/liquid interface. For this, membranes with adjustable pore size are studied: anodically oxidized aluminium (AAO, prepared within this project), ion track-etched polymer membranes, and unmodified and metal coated membrane pores. This is pursued by constant and pulsed high potential electrochemical etching, electrodeposition, cyclic voltammetry and chronoamperometry at porous membranes, surface and cross-sectional characterization (atomic force microscopy, scanning electron microscopy).
PhD student: Niclas Blanc
2nd supervisor: Axel Rosenhahn
Using 0D Confinement in Micelles to Tune Electrochemical Reactivites
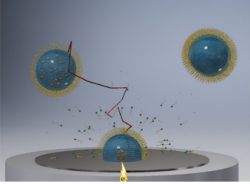
We will study the effects of confining reactants on electrochemical reactions that are relevant for renewable energy technologies, such as the production of green hydrogen. This will be achieved by encapsulating the reacting redox molecules in micelles and varying the micelle size and wall-solute interaction. Novel electrochemical single entity experiments of individual micelle’s at microelectrodes will be used and complemented by classical ensemble studies pf micelles adsorbed on macroelectrodes by various electrochemical techniques, including cyclic voltammetry and rotating disc electrode experiments.
Additional characterization of the reactant-filled micelles will be done by atomic force microscopy, transmission electron microscopy and IR or NMR-spectroscopy, as well as by the many other techniques available at the other members of the GRK.
PhD students: Johanna Angona, Thais Schroeder Rossi
2nd supervisors: Christian Merten, Axel Rosenhahn
Principal Investigator David Van Craen
Catalysis in the confined space of metallocontainers

The aim of the project is to utilize charge-neutral self-assembled metallocontainers as catalysts for enantioselective C-C bond formations. The design is guided by computational models and the ligand synthesis for the formation of metallocontainers is based on a wide range of organic reactions, e.g., ester or ether formations, bromination or substitution reactions, Sonogashira or Suzuki coupling reactions and click chemistry. The container self-assembly is driven by coordination of ligands to suitable metal centers and the properties towards guest uptake and the catalytic activity of the systems is investigated by a broad range of analytical methods.
PhD student: Abhinu Menoth
2nd supervisor: Christian Merten
Former Principal Investigators
Former Principal Investigator Dominik Marx
Nanoconfined Chemistry within Graphene Slit Pores
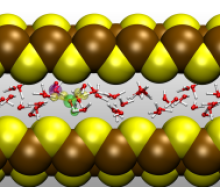
The aim of the project is to explain nanoconfinement effects on chemical reactivity by computationally studying archetypical chemical reactions in water within graphene slit pores via tuning interlayer distances. Confinement is achieved by graphene sheets with different interlayer distance down to the limit of allowing only a water monolayer in the confined space, while water self-dissociation plus other key reactions, e.g. nucleophilic substitutions and elimination reactions, are explored. The employed methods comprise ab initio molecular dynamics simulations (CP2K), DFT with dispersion corrections (RPBE-D3), a QM/MM hybrid scheme combining force fields with ab initio electronic structure calculations, and sampling of free energy landscapes.
PhD students: Saskia Körning, Abhishek Chattopadhyay
2nd supervisors: Lars Schäfer, Kristina Tschulik
Former Principal Investigator Patrick Nürnberger
Exploring photochemical reaction dynamics modified by confining surroundings
This project aimed to determine how encapsulation influences the dynamics of photochemical processes. A special focus was set on the dependence of photoinduced charge transfer on confining conditions, including photoswitchable molecular containers. Unlike homogeneous environments, confining solutions significantly alter the (photo)chemical reactivity leading to changes in the reaction mechanisms and dynamics.
PhD student: Niklas Sülzner
2nd supervisor: Wolfram Sander
Former Principal Investigator Wolfram Sander
Reactive Molecules in Confinement – from Matrix Cages to Surface Molecules
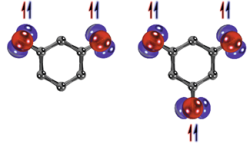
Controlling the reactivity of short-lived intermediates is one of the main routes to increase the selectivity in chemical reactions. The aim of the project is to synthesize and characterize reactive species such as radicals, carbocations, carbenes, or nitrenes in confined environments and to study changes in their reactivity induced by the confinement. Confined spaces to be studied are matrix cages, porous polymeric materials, supramolecular containers, and surfaces. A variety of techniques will be used in this project: low-temperature and time-resolved spectroscopy, EPR, IR, and UV-vis spectroscopy, and synthesis of organic precursor molecules including isotopic labelling.
PhD student: Kseniya Gorbatenko
2nd supervisor: Karina Morgenstern
Reactive Molecules in Confinement – from Matrix Cages to Surface Molecules
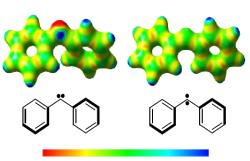
The aim of the project is to synthesize and isolate highly reactive carbenes and radicals in matrix cages, supramolecular host molecules, and on surfaces, and to investigate the bimolecular chemistry of these species. In combination with organic synthesis including isotopic labelling, laser-induced photochemical reactions are studied for carbene precursors and small molecules under cryogenic conditions, e.g. inert gas matrices, amorphous water ice, or parahydrogen matrices. The employed spectroscopic methods combine matrix isolation with IR and UV-VIS studies as well as with X- and Q-band EPR spectroscopy.
PhD student: Julien Rowen
2nd supervisor: Karina Morgenstern
